‘TAM Mild Jalapeno II’: A New, Multiple-virus-resistant,
Mild Jalapeno Pepper
Kevin M. Crosby1 and Benigno
Villalon2
Texas
Agricultural Experiment Station, 2415 E. Highway 83, Weslaco,
TX 78596
Additional index words. Capsicum annuum potyviruses,
resistance, germplasm, open pollinated
The Texas Agricultural Experiment Station (TAES) at Weslaco
announces the release of ‘TAM Mild Jalapeno II’, a
multiple-virus resistant, low-pungency pepper (Capsicum annuum
L.). The project to breed virus-resistant peppers at the TAES
began in 1971. Several cultivars of virus-resistant jalapenos
have been released over the last 20 years (Villalon, 1983; Villalon
et al., 1992, 1994). The production of hot and sweet peppers in
Texas has fluctuated
from 1200 to 7000 ha over the last 30 years. Currently, close
to 1700 ha are cultivated statewide. One of the most persistent
problems on peppers in south Texas
has been virus infection. The two most serious virus pathogens
are tobacco etch virus (TEV) and pepper mottle virus (PepMov),
both transmitted by aphids primarily the cotton aphid (Aphis gossypii
Glover) and the green peach aphid (Myzus persicae Sulzer). Traditionally,
all common jalapeno cultivars grown in south Texas
have been susceptible to local strains of TEV and PepMov. Yield
reductions from infected plants may be as high as 45%, particularly
when fruit is intended for the fresh market (Greenleaf, 1986).
The best solution to the problem is the development of resistant
cultivars with high quality fruit. Multiple sources of single
resistance genes against these potyviruses have been documented
in various germplasm lines (Cook, 1960; Greenleaf, 1956; Kyle
and Palloix, 1997; Zitter and Cook, 1973).
Origin
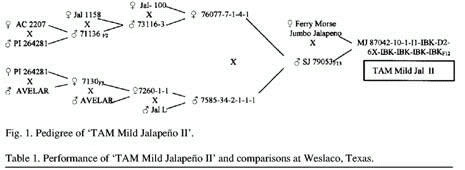
‘TAM Mild Jalapeno II’ (TMJII) originated
from an F12 selection out of the cross of ‘Ferry
Morse Jumbo Jalapeno’ and sweet jalapeno 76053, a line with
resistance to Texas isolates of TEV, PVY, PepMov, and TMV (Fig.
1). This pedigree included three sources of potyvirus resistance,
‘Avelar’, ‘PI 264281’, and ‘AC 2207’
(Cook, 1960; Greenleaf, 1986; Zitter and Cook, 1973), as well
as several jalapeno lines. Field selections were conducted for
more than 12 generations. Progeny of the initial F3 single-plant
selection were placed in isolation blocks for two consecutive
seasons, bulking superior plants. This was followed by another
single-plant selection. Selfed progeny of this plant were then
grown in isolation blocks where superior plant selections were
bulked to carry on the inbreeding. At each generation, seedlings
were mechanically inoculated with Texas
isolates of TEV and PepMov, prior to transplanting to the field,
to verify resistance (Villalon, 1981). Susceptible control cultivars
were included to ensure viral pathogenicity. The final selection
was at the F12 generation and possessed uniform plant and fruit
traits.
Description
‘TAM Mild Jalapeno II’ is adapted to the
high temperatures and virus disease pressures of south Texas.
The concentrated set of large, crack-free fruit on compact plants
makes it ideally suited to once-over harvests and high density
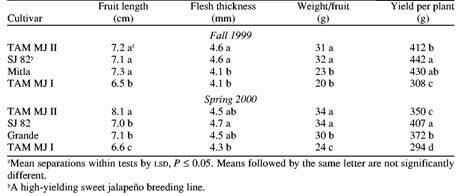
plantings. Comparisons for field performance, yield, fruit quality,
and virus resistance were conducted at the TAES center at Weslaco
during the period from 1996 to 2001. In addition, yield plots
were established in a commercial field in Edinburg,
Texas, during the Fall 2000
season. All plants were grown on a silty, clay loam soil (Weslaco)
or sandy soil (Edinburg),
under commercial practices: transplants, drip irrigation, and
chemical pest control. Plants of both ‘TMJ II’ and
‘Mitla’, inoculated with TEV, PVY, and PepMov, were
transplanted to 10-L pots in the greenhouse and into field plots
to observe effects on plant growth and fruit quality.
In the fall season, ‘TMJ W had significantly larger
fruit and thicker flesh than ‘Mitla’, and similar
yield per plant. In the spring season, fruit size and flesh thickness
were significantly greater than ‘Grande’, but yield
was significantly lower at 350 g/plant compared to 372 g/plant
(Table 1, Fig. 2). In both seasons, ‘TMJII’ matured
5 dbefore ‘Mitla,’ at Weslaco.
In the Fall 2000 season, yields and fruit quality were compared
among ‘TMJ 11’ and the commercial cultivars Mitla,
Grande, and Tula, from a trial on sandy soils in Edinburg (Table
2). Yield of ‘TMJ II’ (435 g/plant) was not significantly
different than the popular cultivar Grande (444 g/plant), but
was significantly less than ‘Mitla’ (502 g/plant).
However, the percentages of cull fruit for both ‘TMJ II’
(12%) and ‘Grande’ (13%) were significantly lower
than the 19% level for ‘Mitla’. In addition, ‘TMJ
II’ fruit produced very little anthocyanin compared to fruit
of ‘Grande’ and ‘Mitla’. This purple-black
pigment is undesirable for fresh-market peppers and reduces the
value of the crop. Total capsaicin concentrations of mature fruits
from ‘TMJ II’ plants grown in Uvalde and College
Station,
Received for publication 30 Oct. 2001. Research conducted at
the Texas Agricultural Experiment Station, Weslaco,
Texas. Use of trade names does
not imply endorsement of the products named nor criticism of similar
ones not named. The authors thank Rick Hernandez, Cory Dombrowski,
Kay Harding, and Alfredo Rodriguez for their valuable help in
conducting greenhouse and field experiments. Thanks also to Dr.
Richard Christie and Dr. John Kao for providing several different
strains of TEV and Dr. Brad Reddick for providing a strain of
PepMov.
1Assistant Professor, corresponding
author: k-crosby@tamu.edu.
2Professor Emeritus.
Texas,
in Spring 2001, were measured by high-performance liquid chromatography
(Hoffman et al., 1983). Fruits from a hot jalapeno cultivar, ‘Grande’,
grown in College Station,
were also analyzed for comparison. Total capsaicin, on a dry-weight
basis, was converted to Scoville Heat Units (SHU) following the
method of Collins et al. (1995). ‘TMJ II’ fruit from
College Station had
higher total capsaicin (108 mg.kg-1,
1620 SHU) than fruit from Uvalde (72 mg.kg
-1, 1080 SHU). By comparison, the hot jalapeno ‘Grande’
had a capsaicin concentration of 1332 mg.kg
-1 (19980 SHU).
In both greenhouse and field plots, TEV inoculated plants
of ‘Mitla’ had small, misshapen fruit, and chlorotic
leaves, while inoculated plants of ‘TMJ II’ had normal
fruit and no chlorosis (Fig. 3). None of the plants appeared to
be reduced in height. PepMov inoculated plants of ‘Mitla’
exhibited severe leaf distortion, plant stunting, and reduced
yield of misshapen fruit. ‘TMJ II’ plants inoculated
with PepMov exhibited mild chlorosis on older leaves but no distortion
or misshapen fruit. No symptoms were observed on ‘TMJ II’
or ‘Mitla’ after inoculation with PVY. ELISA tests
were conducted on leaf samples from inoculated plants of ‘TMJ
II’ and ‘Mitla’ to determine the presence of
TEV, PepMov, and PVY. Leaves from ‘Mitla’ tested positive
for TEV and PepMov with absorbance readings of 1.70 and 2.21,
respectively, but negative for PVY (0.021). Leaves from ‘TMJ
II’ tested negative for TEV (0.047) and PVY (0.019) and
weakly positive for PepMov (0.49). Susceptible control bell pepper
leaves tested positive for TEV (4.0) and PepMov (2.37).
The large, low-pungency fruit of ‘TMJ II’
will make it equally suited for fresh-market and processing uses.
The multiple-virus resistance and adaptation to high temperatures
will be valuable attributes in regions where these stresses exist.
Availability
Breeder’s seed will be maintained by the Texas
Agricultural Experiment Station at Weslaco.
Application for plant variety protection is being filed for ‘TMJ
II’. This cultivar may be licensed through the Texas Agricultural
Experiment Station, for commercial seed production.
Literature Cited
Collins, M.D., L.M. Wasmund, and P.W. Bosland. 1995.
Improved method for quantifying capsaicinoids
in Capsicum using high-performance liquid chromatography. HortScience
30:137-139.
Cook, A.A. 1960. Genetics of resistance in Capsicum annuum
to two virus diseases. Phytopathology 50:364-367.
Greenleaf, W.H. 1956. Inheritance of resistance to tobacco
etch virus in Capsicum frutescens and in Capsicum annuum. Phytopathology46:371-375.
Greenleaf, W.H. 1986. Pepper breeding, p.67-134. In: M.J. Basset
(ed.). Breeding vegetable crops. AVI, Westport,
Conn.
Hoffman, P.G., M.C. Lego, and W.G. Galetto.1983.
Separation and quantification of red pepper
major heat principles by reverse-phase high-pressure liquid chromatography.
J. Agr. Food Chem. 31:1326-1330.
Kyle, M.M. and A. Palloix.1997. Proposed revision of
nomenclature for potyvirus resistance genes in Capsicum. Euphytica
88:231-239.
Villalon, B. 1981. Breeding peppers to resist virus diseases.
Plant Dis. 65:557-562.
Villalon, B. 1983. TAM mild jalapeno pepper-1
pepper. HortScience 18:492-493.
Villalon, B., F.J. Dainello, and D.A. Bender. 1992. ‘TAM
Veracruz’
hot jalapeno pepper. Hort Science 27:184-185.
Villalon, B., F.J. Dainello, and D.A. Bender. 1994. ‘Jaloro’
hot yellow jalapeno pepper. HortScience 29:1092-1093.
Zitter, T.A. and A.A. Cook. 1973.
Inheritance of tolerance to a pepper virus in
Florida.
Phytopathology 63:121-1212.
HORTSCIENCE, VOL. 37(6),
OCTOBER 2002
CULTIVAR & GERMPLASM RELEASES
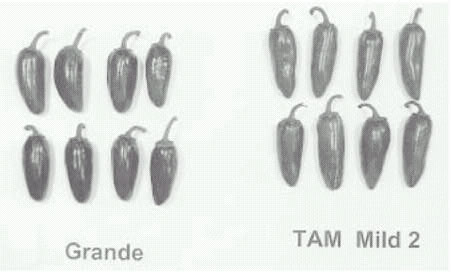
Fig. 2. Fruit of ‘Grande’ and ‘TAM
Mild Jalapeno II’.
| Table 2. Performance |
of ‘TAM Mild Jalapeno |
II’ and comparisons at |
Edinburg, Texas, Fall |
2000. |
| |
Fruit length |
Weight/fruit |
Yield per plant |
Culls |
| Cultivar |
(cm) |
(g) |
(g) |
(%) |
| TAM MJ 11 |
8.0 az |
33 ab |
435 b |
12 b |
| Mitla |
7.0 c |
24 c |
502 a |
19 a |
| Grande |
7.5 b |
31 b |
444 b |
13 b |
| Tula |
7.5 b |
36 a |
371 c |
6 c |
| TAM MJI |
6.5d |
21c |
315d |
12b |
| Mean separations by |
LSD, P < 0.05. Means followed by the same letter
are not significantly |
different. |

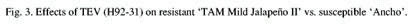
|



