|
Improvement
of Seedling Emergence of Lupinus texensis Hook. Following Seed
Scarification Treatments
Tim D. Davis1, Steven
W. George2, Abha Upadhyaya3, and Jerry Parsons4
Texas A&M University Research and Extension Center
Texas Agricultural Experiment Station and Extension Service
17360 Coit Rd.
Dallas, TX 75252-6599
Received for publication 16 July 1990; in revised form September 20, 1990. Contribution
No. 25726 from the Texas Agricultural Experiment Station.
1. Associate Professor of Ornamental Horticulture.
2. Extension Landscape Horticulture Specialist.
3. Research Associate.
4. Extension Horticulturist, current address: Texas
Agricultural Extension Service, 1143 Coliseum Road, San
Antonio, TX 78219.
Abstract: Seeds from four commercial seedlots
of Lupinus texensis Hook. (Texas bluebonnet) were
placed in concentrated sulfuric acid for 0 to 120 minutes and
then sown. Emergence was promoted by acid scarification in
three of the four seedlots. For the lots that responded to
acid scarification, the optimal scarification time was 30-60
minutes which resulted in 85-95% emergence one month after
planting. In addition to increasing the total number of
seedlings that emerged, acid scarification hastened
emergence. The same aliquot of sulfuric acid was used for
five 60-minute scarification periods before its efficacy was
reduced. Acid scarification did not reduce seed coat
thickness or strength but created several small pores in the
seed coat which likely facilitated imbibition. Cutting,
filing, or piercing the seed coat promoted emergence to a
similar extent. Placement of seeds in 85°C
(185°F) water and then cooling for 24 hrs promoted
emergence relative to the non-treated controls, but was not
as effective as other scarification techniques. Freezing and
thawing of seeds had no effect on emergence. Results indicate
that acid scarification functions by removing a mechanical
rather than a chemical barrier to germination
of L. texensis.
Index words: germination, native plants, seed propagation, sexual
propagation, sulfuric acid, Texas bluebonnet
Significance to the Nursery Industry
Lupinus texensis is a potentially useful low maintenance annual
but, as with other newly-domesticated species, propagation may be
an obstacle to further development. Our findings indicate that considerable
seedlot variability exists with regard to the need for sulfuric acid
scarification. Growers should test the response of small seedlot samples
to acid scarification before deciding on the length of the acid scarification
period. If this is not possible, then a 45-minute acid treatment should
promote emergence in most seedlots of L. texensis without causing
significant damage to the sensitive lots. A given quantity of sulfuric
acid can be used for at least five scarification treatments before
its efficacy is reduced. Acid scarification works by removing a physical
barrier to germination. Thus any treatment that produces a small pore
in the seed coat will likely improve emergence.
Introduction
Lupinus texensis Hook. (Texas bluebonnet) is an
attractive spring flowering annual native to Texas that has
considerable potential for use as a low maintenance bedding
plant or for use in roadside plantings. The species is
adapted to a variety of environmental conditions and has been
grown successfully in many areas of the world (1). Being a
nitrogen-fixing legume, L. texensis requires little or
no nitrogen input on a variety of Texas soils. Furthermore,
the plant requires little irrigation on most sites and few,
if any, pesticide applications. Because of these
characteristics, there is considerable interest in commercial
production of this species. In fact, over 270,000 transplants
were sold by North Central Texas retail outlets during fall
1989 (2). In addition, a large amount of packaged seed was
purchased for highway seeding projects and home landscape
use.
An obstacle to the further development and commercial
production of L. texensis is variability in seed
germination and emergence. As with many other native species,
growers have experienced problems in obtaining uniform
emergence during greenhouse production. Because of the hard
seed coat, scarification is required to obtain a high
percentage of seedling emergence in a reasonable period of
time. There is, however, currently no information on the
optimal length of time for sulfuric acid scarification of
seed of L. texensis. Optimum sulfuric acid
scarification times vary from a few minutes in some species
(3) to several hours in others (4). It is also possible that
considerable seedlot-to-seedlot variability within a species
may exist in response to seed scarification treatments.
The objectives of the current investigation were to
determine:
- 1) the response of four commercial seedlots
of L. texensis to a range of sulfuric acid
scarification times;
- 2) how many times a given aliquot of sulfuric acid can
be used before its scarification efficacy is reduced;
- 3) if acid scarification works primarily by removing
the physical restraint to germination (i.e. the hard seed
coat); and
- 4) the efficacy of a variety of mechanical
scarification techniques in promoting seedling emergence
of L. texensis.
The development of mechanical scarification techniques would
be desirable because the corrosive nature of sulfuric acid
creates a potentially hazardous situation in the workplace.
Materials and Methods
Four commercial seedlots (designated A, B, C, and D) were
obtained for use in the study. Some physical characteristics
of the seedlots are given in Table 1. Four separate
experiments were conducted:
- 1) acid scarification time experiment --
seeds from each lot were placed in concentrated
(36 N) sulfuric acid (about 60 seeds per 50 ml) for 0, 15,
30, 45, 60, 75, 90, or 120 min. The seed was then rinsed with
distilled water several times before sowing;
- 2) repeated use of acid experiment -- seeds from
lot A were placed for 60 minutes in the same aliquot of
sulfuric acid (about 60 seeds per 50 ml) that had been
previously used zero to six times for 60 minute acid
scarification treatments;
- 3) mechanical scarification experiment -- seed
from each lot was left non-treated (control), placed in
concentrated sulfuric acid for 60 minutes, cut through
the seed coat with a razor blade, or rubbed against a
metal file until visible seed coat disturbance occurred;
and
- 4) other scarification treatment experiment --
seeds from lot A were
- a) left untreated (control),
- b) lightly tapped using a hammer and nail to
create a small hole in the seedcoat,
- c) placed in tap water, frozen
and thawed one time before planting,
- d) placed in tap water, frozen
and thawed three times before planting,
- e) soaked in room temperature
[22°C, (72°F)] tap water for 24 hr, and
- f) placed in 85°C (185°F)
tap water which was allowed to cool for 24 hr.
Table 1. Fresh weight, volume, and density of the four
commercial seedlots of L. texensis used in the
scarification experiment.
| | Seedlot |
| Characteristic |
A |
B |
C |
D |
| Wt./100 seeds (g) |
3.62 az |
3.60 a |
3.73 a |
2.34 b |
| Vol./100 seeds (cm3) |
2.70 a |
2.70 a |
2.70 a |
1.70 b |
| seed density (g/cm3) |
1.34 a |
1.33 a |
1.38 a |
1.38 a |
z Means within a row with common lower case letters are not significantly
different at the 5% level of probability (n = 3).
After the seed treatments were administered, the seed
was planted 1/8 in. deep in 27 x 53 cm (11 x 21 in) plastic flats containing
a medium of peat:perlite (1:1 by vol). The flats were placed in an unshaded
greenhouse (day/night temperature regime of about 27/20°C or 81/68°F)
and emergence was evaluated after one week and again after one month.
For
the acid scarification experiment, seeds from the different seed lots
that had been placed in acid for 0 or 90 minutes were cut in half and
seed coat thickness was measured using a dissecting microscope. Seed
coat strength was measured by placing the seed in a Carver Laboratory
Press and determining the force required to crack the seed coat. Also,
seeds from lot A that had been left in acid for 180 minutes were photographed
under a dissecting microscope to document the seed coat lesions caused
by acid scarification.
All experiments were conducted at least twice
utilizing a randomized complete block experimental design. The number
of seeds per treatment is given in the respective tables or figures.
Statistical inferences were made based upon 95% confidence limits after
calculation of z values (8).
Results and Discussion
There was considerable seedlot-to-seedlot variability with
regard to the need for acid scarification. Only 16 and 23%
of the non-scarified seeds emerged after one month for the A
and C lots, respectively (Table 2). In contrast, 50 and 71%
of the non-scarified seeds emerged after one month for the B
and D lots, respectively. For the D lot, acid scarification
for any length of time did not significantly increase
seedling emergence compared to non-scarified seed. In
contrast, acid scarification promoted emergence in the
remaining seedlots. For lots B and C, a 30 minute
scarification period was sufficient for obtaining optimum
emergence after one month; with lot A, 45 min. was
needed. The highest percent emergence obtained in the acid
scarification experiment ranged from 80% in lot D to 95% in
lot A. Placement of seed in the acid for 120 minutes reduced
emergence compared to the 45 min. treatment in all
seedlots. The most dramatic decline occurred with lot D which
only had 28% emergence after the 120 min. scarification
treatment compared to 80% emergence for the 45
min. treatment. Lot D had the smallest seed size (Table 1)
and apparently is quite susceptible to damage from sulfuric
acid.
These results demonstrate the need for testing small
samples of seed from individual seedlots of L. texensis seedlots
before deciding on the length of time needed for acid scarification.
If this is not possible, our data suggest that a 45 minute treatment
will be effective in promoting emergence in most seedlots of L. texensis,
yet safe for lots that are highly sensitive to sulfuric acid (e.g.
lot D).
Table 2. Percent seedling emergence of L. texensis after one
week and after one month following sowing of seeds placed
in concentrated sulfuric acid for varying lengths of time.
| |
Acid scarification time (min.)
|
| Seedlot |
0 |
15 |
30 |
45 |
60 |
75 |
90 |
120 |
| |
One month
|
| A |
16 jz |
24 ij |
75 def |
95 a |
93 ab |
93 ab |
84 c |
84 c |
| B |
50 g |
68 f |
86 c |
87 bc |
83 cd |
87 bc |
83 cd |
71 ef |
| C |
23 ij |
44 gh |
94 ab |
94 ab |
84 c |
86 c |
75 def |
72 ef |
| D |
71 ef |
74 ef |
79 cde |
80 cde |
69 f |
54 g |
43 h |
28 i |
| |
One week
|
| A |
0 o |
7 n |
41 i |
58 cdef |
72 a |
73 a |
65 abcd |
61 bcdef |
| B |
13 mn |
29 j |
66 abc |
70 ab |
61 bcdef |
64 abcd |
63 abcde |
55 def |
| C |
8 n |
16 lm |
65 abcd |
59 cdef |
71 ab |
68 abc |
68 abc |
51 fgi |
| D |
24 jkl |
41 i |
53 efg |
67 abc |
55 def |
44 gi |
42 i |
27 jk |
|
zPercentages with common lower case letters are not significantly
different at the 5% level of probability (n = 120).
|
Although acid scarification of lot D did not improve percent
emergence after one month, it did speed emergence. One week
after planting only 24% of the non-scarified lot D seed had
emerged whereas emergence was significantly higher in the 15,
30, 45, 60, 75, and 90 minutes treatments (Table 3). The
highest emergence percentage after one week (67%) occurred in
the 45 minute acid treatment. Similarly, all other seedlots
showed improved emergence at one week in response to acid
scarification. Thus, in addition to increasing the total
number of seedlings that emerged, acid scarification also
hastened emergence.
Table 3. Percent seedling emergence one week and one month
after planting of L. texensis seed subjected to
different scarification treatments.
| |
Treatment |
| Seedlot |
Control |
Acid |
Cut |
Filed |
| |
One month |
| A |
8 ez |
85 b |
80 b |
98 a |
| B |
40 cd |
85 b |
65 bc |
100 a |
| C |
20 de |
85 b |
80 b |
85 b |
| D |
80 b |
80 b |
65 bc |
50 c |
| |
One week |
| A |
5 f |
65 bcd |
80 bc |
98 a |
| B |
10 f |
85 b |
65 bcd |
100 a |
| C |
5 f |
45 de |
80 bc |
80 bc |
| D |
35 e |
65 bcd |
60 cd |
45 de |
|
zPercentages with a common lower case letter are not significantly
different at the 5% level of probability (n ≥ 20).
|
The same aliquot of sulfuric acid was used for five 60 minute
scarification treatments before any significant change in
efficacy was detected (Fig. 1). This was despite the fact
that considerable change in the appearance of the acid
occurred after repeated use. After being used for three
60 minute scarification treatments the acid was very dark in
color, presumably due to the extraction of unknown compounds
from the seed coat. The consistency of the acid also changed
after repeated use. Acid used several times became more
viscous than unused acid.
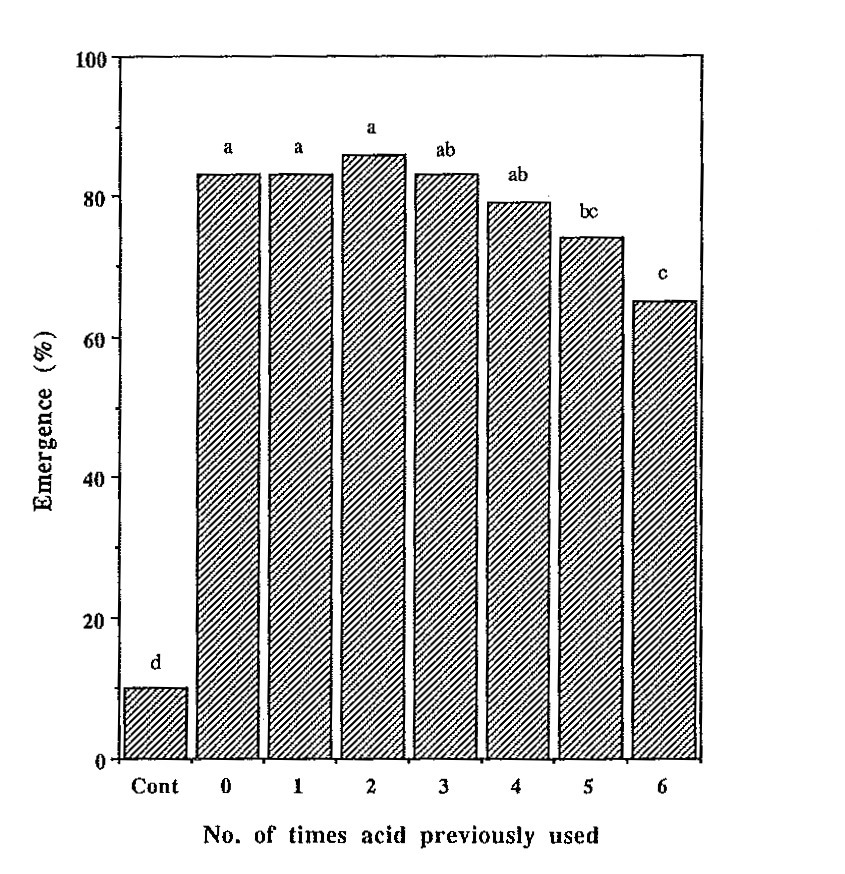
Fig. 1. Percent seedling emergence of L. texensis one month after
planting seeds of lot A placed in sulfuric acid that had been
previously used for zero to six 60 minute scarification treatments.
Control seed was not treated with acid.
Percentages with common lower case letters are not significantly
different at the 5% level of probability (n = 80).
Despite these rather dramatic changes in physical properties,
emergence was still 65% when the acid was used six times
previously compared to 83% when not used previously. Thus,
the common practice of discarding acid after one or two
scarifications may not be important
for L. texensis. Apparently, contact with seed coats
of L. texensis does not substantially reduce sulfuric
acid strength until after at least five or six scarification
treatments.
Seed coat thickness and strength did not differ among
seedlots and were unaffected by the 90-minute acid
scarification treatment (data not presented). This was an
unexpected result because acid scarification is generally
thought to decrease seed coat thickness and strength
(5). Sulfuric acid created several small,
randomly-distributed pores in the seed coat
of L. texensis (Fig. 2). These areas of the seed coat
apparently are more susceptible to acid hydrolysis than the
remaining portion of the seed coat. Although the small pores
did not measurably affect seed coat strength, they probably
served as channels for water uptake during imbibition. Thus,
acid scarification seems to promote germination and emergence
of L. texensis by facilitating water uptake through
small localized areas rather than by causing a uniform
thinning of the seed coat.
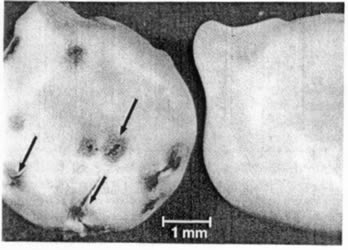
Fig. 2. Photograph of (left) acid-scarified (180 minutes) and (right) non-treated seeds
L. texensis (18 x).
Cutting the seed coat with a razor blade or rubbing the seed
against a file improved emergence compared to the non-treated
seeds in lots A-C (Table 4). With lot D, cutting the seed
coat had no effect whereas filing the seed reduced emergence
to 50%. The reason for this response is not clear, but may be
related to the small size of the seed in lot D. The pressure
exerted on the seed during filing may damage the embryo. For
lots A-C, it appears that any treatment that creates a weak
area or opening in the seed coat will improve germination and
emergence. This suggests that acid scarification improved
emergence by removing a physical barrier (i.e. the hard seed
coat that is impermeable to water) rather than by removing a
chemical inhibitor from the seed. This is similar to what has
been observed for seed of some other species (6) although
with seeds of Panicum coloratum, acid scarification
destroys a germination inhibitor (9).
In addition to promoting total emergence, cutting or filing
the seed also hastened emergence in lots A-C. One week after
sowing, these treatments clearly promoted emergence relative
to the control (Table 4). Nearly all of the cut or filed seed
that emerged during the experiment did so during the first
week. In contrast, only 5-10% of the non-scarified seeds had
emerged by this time. With lot D, cutting the seeds improved
emergence after one week but filing had no effect.
Table 4. Percent seedling emergence of L. texensis seeds
(lot A) subjected to several different treatments.
| |
Treatment |
| Time after emergence |
Control |
Holez |
1 freeze thaw cycle |
3 freeze thaw cycles |
Room Temp. H2O |
Hot H2O |
| 1 week |
0 cy |
80 a |
3 c |
3 c |
5 c |
23 b |
| 1 month |
13 c |
80 a |
15 c |
13 c |
18 c |
38 b |
|
zhole created in seed coat using a small nail.
|
|
yPercentages within a row with common lower case letters are
not significantly different at the 5% level of probability (n = 40).
|
Because lot A seemed to benefit most from scarification, a
variety of treatments that have been reported to promote
germination of hard-seeded species was evaluated for this
seedlot. Piercing the seed coat with a nail strongly promoted
emergence after one week and after one month compared to the
control (Table 4). This further supports our conclusion that
scarification works by creating small channels for water
uptake. A single, small pore in the seed coat apparently is
sufficient for adequate imbibition. The hot water treatment
increased emergence, but to a much lesser extent than the
nail treatment. A similar observation was noted with
Sapindus drummondii seed where a hot water treatment improved
emergence relative to the non-treated control, but less so than other
scarification treatments (7). Soaking the seeds of L. texensis
in room temperature water for 24 hours had no effect on emergence
(Table 4). Likewise, freezing and thawing of the seed had no effect
on emergence. Apparently the freeze-thaw action was insufficient for
creating channels for water uptake.
Based upon the results of this study, it appears that any
treatment that creates a small weakening or opening in the
seed coat of L. texensis will be useful for
increasing seedling emergence. Although sulfuric acid
scarification is an effective treatment for improving
emergence, it is hazardous to use. The mechanical
scarification treatments used in this study were very
effective in promoting emergence, but are too laborious to be
practical on a large scale. It is possible, however, that
mechanical scarifiers can be developed or adapted (e.g. like
those used for alfalfa and clover) which will be useful for
promoting emergence and hence facilitating the commercial
production of transplants of L. texensis.
Literature Cited
- Andrews, J. 1986.
The Texas Bluebonnet. Univ. Texas Press, Austin.
- Anonymous. 1990.
New bluebonnet market extends to home landscape. Texas Horticulturist,
March 1990, p. 10.
- Dehgan, B. and B. Schutzman. 1983. Effect of
H2SO4 and GA3 on seed
germination of Zamia furfuracea. HortScience
18:371-372.
- Dirr, M.A. and C.W. Heuser Jr. 1987. The
Reference Manual of Woody Plant Production: From Seed to Tissue Culture.
Varsity Press, Athens, GA.
- Hartmann, H.T. and D.E.
Kester. 1983. Plant Propagation Principles and Practices.
4th Ed. Prentice-Hall, Englewood Cliffs, NJ.
- Marousky, F.J. and
S.H. West. 1988. Germination
of bahiagrass in response to temperature and scarification. J. Amer.
Soc. Hort. Sci. 113:845-849.
- Munson, R.H. 1984. Germination of western
soapberry as affected by scarification and stratification. HortScience
19:712-713.
- Steele, R.G.D. and J.H.
Torrie. 1960. Principles and Procedures of Statistics. McGraw-Hill,
New York, NY.
- Tischler, C.R. and B.A. Young. 1983.
Effects of chemical and physical treatments on
germination of freshly harvested klein grass (Panicum coloratum)
seed. Crop Sci. 23:789-792.
|



