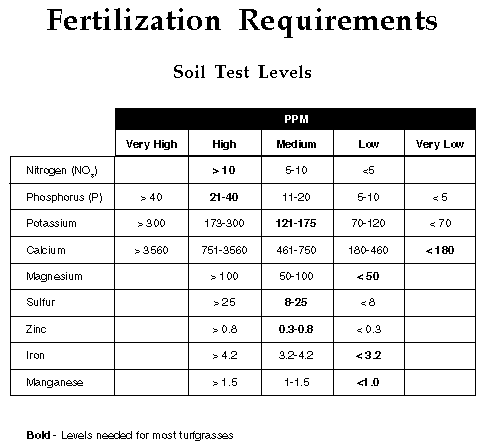
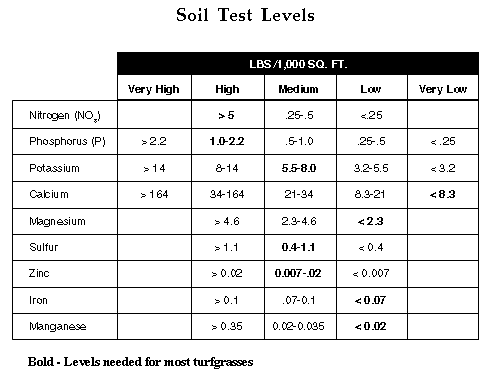
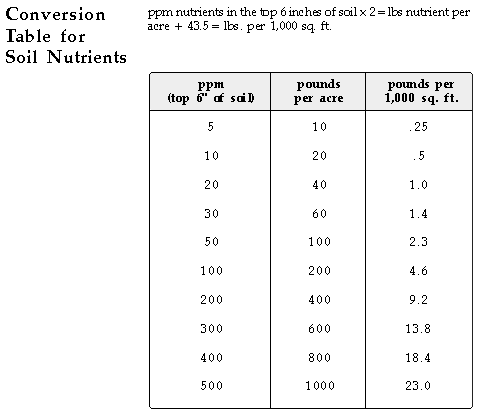
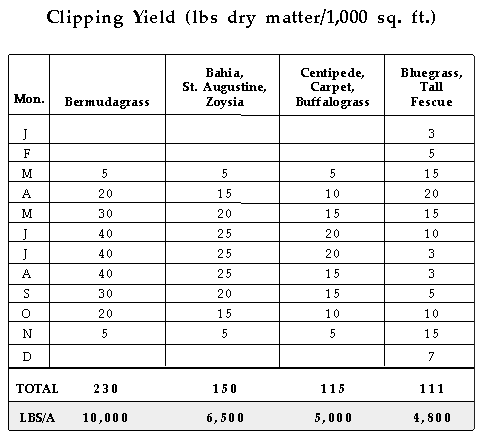




Total fertilizer nutrients needed by turfgrasses assuming all clippings
are removed may be calculated by substracting the soil levels reported from
the total needed. For example, a bermudagrass turf on a soil with 10 ppm
(@ .5 lbs. per 1,000 sq. ft.) phosphorus needs an additional 2 pounds per
1,000 sq. ft. of phosphorus during the growing season. Likewise, if the
soil was reported to contain 120 ppm (5.5 lbs. per 1,000 sq. ft.) potassium,
an additional 2.2 lbs. per 1,000 sq. ft. would be needed during the growing
season. However, if the soil was reported to contain 175 ppm or more potassium,
additional potassium would not be needed.
To effectively use this information, soil tests need to be conducted at
least on an annual basis. Intensively managed turf areas such as golf greens,
need semi-annual soil tests. Soil tests not only identify nutrient deficiencies,
but they can also show an accumulation of nutrients.
For example, phosphorus tends to accumulate following repeated applications
of phosphorus containing fertilizers. Even though phosphorus is removed
by turfgrasses, it is also released to the soil from minerals and organic
matter. Thus, applications of phosphorus may lead to excessive levels of
the nutrients. Soil tests could confirm the presence of these excessive
phosphorus levels.
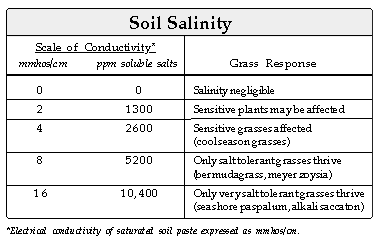
Indirect effects of salts on plants include desiccation, deterioration
of soil physical conditions and an imbalance of plant nutrients. Grasses
are generally injured by one or more of these indirect effects.
The first visible symptom of salt injury is stunted appearing plants - reduced
growth rate, short leaf blades and short internodes. In grasses, leaf growth
decreases linearly with increasing salt levels after reaching a threshold
level; while root growth usually increases at moderate salt levels, then
decreases sharply as salinity increases. A typical growth response of bermudagrass
to increasing salinity would appear as follows:
How Salt Problems Develop. Salt problems usually develop because of poor drainage, high ground water tables and poor quality (salty) irrigation water. Where soils are poorly drained because of an impermeable layer or impermeable topsoil, salts accumulate in the surface soil. Where high ground water tables are present, salts move upward with the water through the finer
capillary pores and accumulate as water evaporates. In clay soils, salts
have been known to accumulate 20 to 30 feet above a water table over a long
period of time.
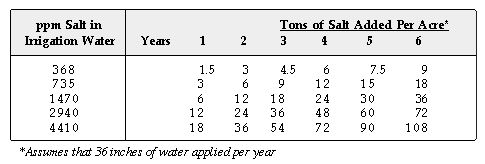
Most salt problems develop, however, directly from salts added by the irrigation
water. This problem usually develops over a long period of time because
large amounts of salt must accumulate before salts affect the growth of
grasses. The amounts of salt added to a soil by irrigation waters over
a period of years when 36 inches of water are applied per acre per year
are shown in the following table.
Water containing 735 ppm soluble salts is considered good quality irrigation
water, yet in several years enough salt would be added to affect most plants.
Thus, salts must be removed by leaching before they accumulate and become
a problem.
Management of Saline Soils. Proper irrigation management (occasional leaching)
and adequate drainage are essential to prevent salinity problems. The only
way to remove salts from the soil is by leaching them below the rootzone.
In areas with adequate rainfall, leaching may not be required. But, in
arid climates periodic leaching by applying excessive irrigation water is
necessary to prevent salinity problems. Where restrictive soil layers prevent
the downward movement of water, lateral tile drains installed directly above
this layer are needed.
To leach salts below the rootzone, "extra" water is needed beyond
that required to "wet" the rootzone. The amount of the "extra"
water needed to leach salts increases with turfgrass sensitivity and with
the salt content of the water. The percentage "extra" water can
be approximated from the following table:
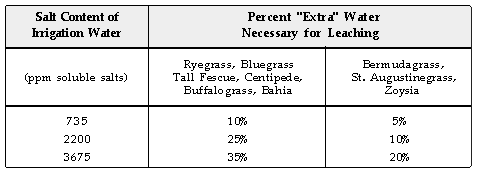
To effectively use the approximation of "extra" water needed
for leaching salts, the turf manager must know the salt content of the water
and the amount of water needed to wet the rootzone. The later value can
be estimated from the following table:
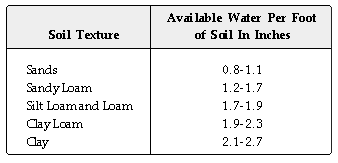
For example, a tall fescue lawn growing on a sandy loam soil irrigated
with water containing 2,000 ppm soluble salts would need the following amount
of water to leach salts below a 6 inch deep rootzone.
From the above table, 12 inches of a sandy loam soil would hold approximately
1.5 inches of available water following irrigation. During the summer this
amount of water would be gone in 5 to 6 days. To effectively leach salts
below the 12 inch rootzone, 1.5 inch of water plus 23% of 1.5 inch, or 1.85
inch, should be applied during the next irrigation. If the lawn is irrigated
every other day, 0.62 inches of water is needed (.5 inch replacement water
plus 0.12 inch (23%) of "extra" water.
Such a watering practice would be "wasteful" of water, but there
are no other means of removing salts from the rootzone during periods of
limited rainfall.
Where restrictive layers develop in the rootzone, cultivation or aeration
may be required before attempting to leach salts through the soil. Deep-tine
aeration is an effective way to improve water movement through a layer in
the top 10 to 12 inches of the rootzone. Such a procedure may need to be
repeated several times each year to prevent salt problems.
When sodium constitutes a significant amount of the salts found in soil
or in the irrigation water, additions of gypsum or sulfur may be necessary.
The calcium in gypsum, or in the gypsum produced by the addition of sulfur,
repalces the sodium on the soil particles and allows water to move the sodium
below the rootzone. Soil tests will indicate the need for amendments such
as gypsum and sulfur.
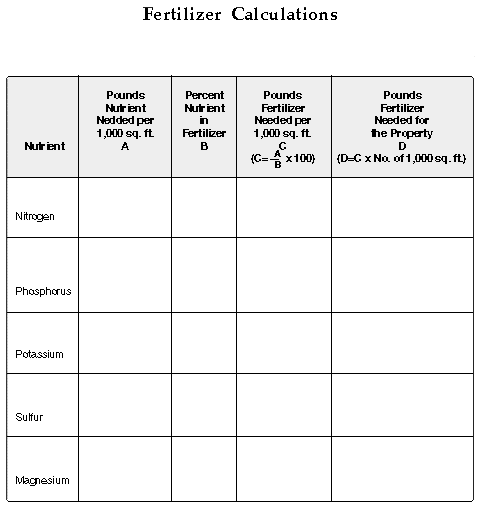
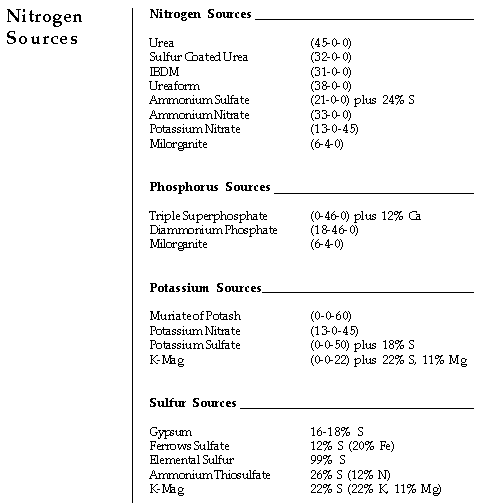

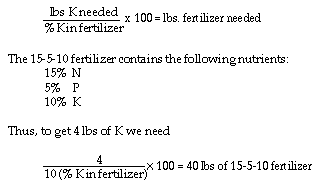
In addition to 4 lbs. K and 2 lbs. P, 40 lbs. of 15-5-10 fertilizer also
provides 6 lbs. of N (40 x 15/100). Therefore, additional nitrogen is not
needed to meet the fertilizer recommendation. Applications of 10 lbs. 15-5-10
fertilizer per 1,000 sq. ft. in March, June, August and October would provide
1.5, .5 and 1.0 lbs. of N, P and K per 1,000 sq. ft. per application. Although
that schedule was not exactly meet the recommendations made for a bermudagrass
athletic field, it would be satisfactory if at least 50% of the nitrogen
in the 15-5-10 fertilizer was slow release.
Fertilizer Requirements (cont.)
Assume the same set of conditions with the folowwing fertilizer sources
available:
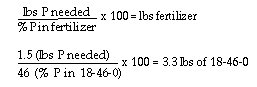
Remember, the 18-46-0 fertilizer provides 18% nitrogen in addition of
46% phosphorus. Thus, 3.3 lbs. of 18-46-0 fertilizer provides 0.6 lbs.
(3.3 x 18/100) of nitrogen. The remaining nitrogen (6-0.6), or 5.4 lbs.,
must be provided by sulfur coated urea.
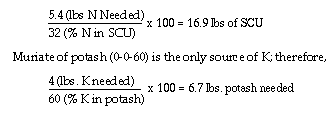
These amounts (3.3 lbs. of 18-46-0, 16.9 lbs. SCU and 6.7 lbs. of potash)
are needed per 1,000 sq. ft. per year. To determine the total amount needed
for the athletic fields, multiply these numbers by the number of 1,000 sq.
ft. to be fertilized. For 60,000 sqq. ft. (the typical area of a football
field), multiply those numbers by 60.
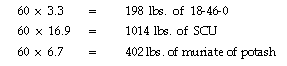
Therefore, for a typical football field, you would purchase 200 lbs.
of 18-48-0, 1,000 lbs. of sulfur coated urea and 400 lbs. of muriate of
potash to meet the 6.1-5.40 fertilizer recommendation.
Soil acidity is determined by a pH meter that measures the hydrogen ion
concentration in the soil solution. The hydrogen row concentration is expressed
in pH units with 5 being strongly acid, 7 being neutral and 8.2 being strongly
alkaline. Each pH unit decrease below 7 represents a 10-fold increase in
hydrogen concentration, or acidity.

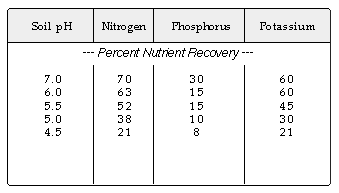
Strongly acid soils reduce the effectiveness of some fertilizer nutrients,
inhibit microbial activity, inhibit the decomposition of thatch and reduce
the effectiveness of some herbicides. The following table shows the reduction
in nutrient recovery by turfgrasses as soil acidity increases (or soil pH
decreases):
Liming Soils to Correct Soil Acidity. The amount of limestone CaCO3)
needed to neutralize soil acidity is based on soil pH and soil texture.
In general, the amount of limestone needed increases as soil pH decreases
and as soil texture changes from sands to loams to clays. The following
table can be used as a guideline to estiamte the pounds of limestone needed
per 1,000 sq. ft. of turf:
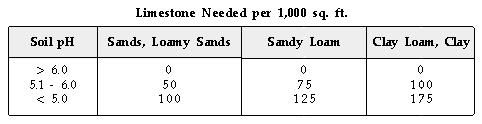
Benefits of Liming Acid Soils